Background
The COVID-19 pandemic has created widespread anxiety among population and fuelled demand for possible cures.
Some Member States authorities have observed that more and more products sold via the internet are being advertised as having a positive effect on the immune system or as protection against infection by coronavirus.
Scientific evidence so far does not support any claim that any food or food supplement protects against COVID-19 infection.
Criminals and fraudsters are using the COVID-19 pandemic as a business opportunity. Products claiming to prevent and cure COVID-19 are being marketed illegally and may even pose significant risks to health.
Health claims made on foods are authorised by the European Commission, following a scientific assessment of the European Food Safety Authority (EFSA).
To date, there are no authorised health claims for a food or food supplement as protecting against viral infection or boosting immunity against any virus.
However, the European Commission has authorised health claims that refer to the role of certain essential nutrients, for example vitamins C and D and iron, in contributing to the normal functioning of the immune system.
These essential nutrients are found in a number of wholesome and nutritious foods and are key to strengthening our body’s immune system as part of a healthy and balanced diet.
Observed sales of products claiming to prevent and cure COVID-19 has led to action by some Member States’ authorities, namely through alerting some market places about products and product advertisements on their platforms regarding dubious and illicit practices or processing actions and investigations at national level.
In view of this, to facilitate common and coordinated approach to this issue across the EU, the European Commission proposed a coordinated action plan on online offers and advertising of food related to COVID-19.
Action:
In coordination with EU countries authorities, the European Commission has called Member States to reinforce their vigilance and adapt their control activities on online offers and advertising of food related to COVID-19. The Commission has asked Member States authorities to:
- Trace and identify websites, sellers and operators with illegal practices in the marketing of food and food supplements linked with COVID-19 sold online in the European Union
- Follow-up on non-compliances and suspicions of fraudulent practices that are identified
- Strengthen the cooperation and administrative assistance between Member State authorities on the control of internet sales
Inform consumers that products bearing COVID-19 infection-related health claims are illegal and might even be injurious to their health.
Timeline and Reporting
|
Activity |
Time |
|
Launch event with Member States competent authorities |
13-17 April 2020 |
|
Start of the action |
20 April 2020 |
|
Reporting to the Commission and communication |
|
Results
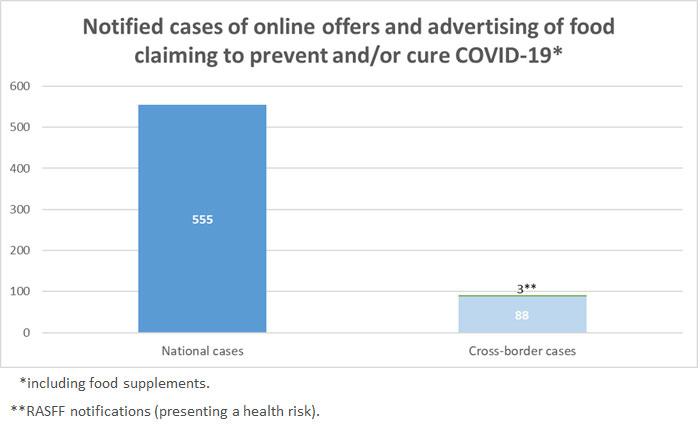
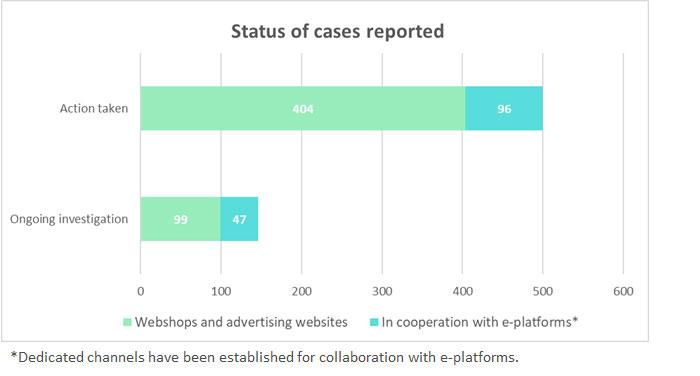
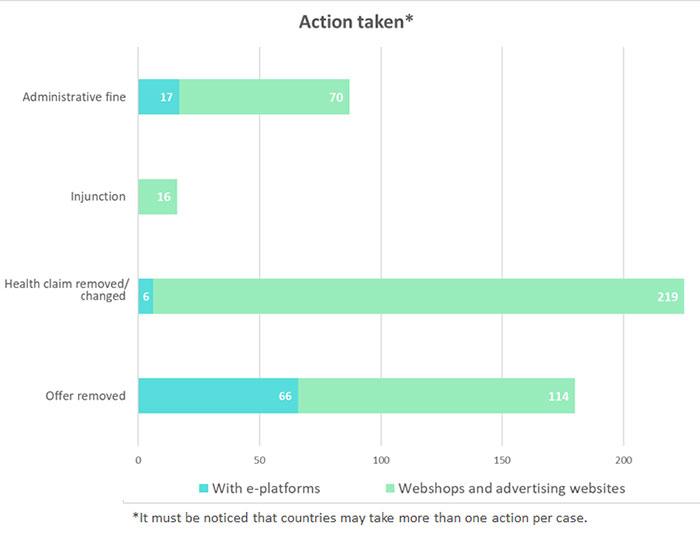
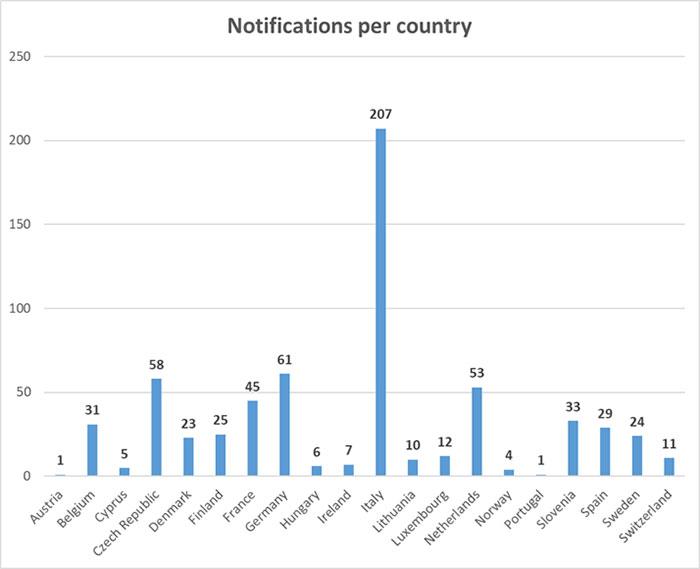
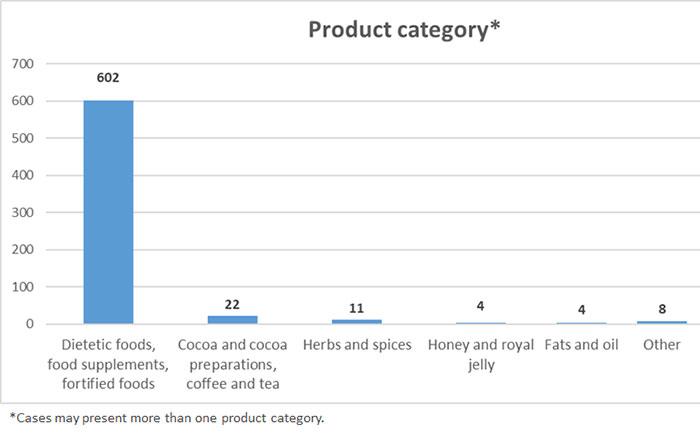
Member States competent authorities shall report the Commission the results of their official controls.
These figures will be regularly published here (updated 6/7/2021)
Contact: SANTE-ECOMMERCE ec [dot] europa [dot] eu (SANTE-ECOMMERCE[at]ec[dot]europa[dot]eu)
ec [dot] europa [dot] eu (SANTE-ECOMMERCE[at]ec[dot]europa[dot]eu)
Press releases
Legal Basis for the action plan
In the EU, it is forbidden to attribute medicinal properties to food. In particular, Article 7 of Regulation (EU) No 1169/2011 stipulates that food information shall not attribute to any food the property of preventing, treating or curing a human disease, nor refer to such properties.
This applies also to any reference made to COVID-19.
In addition, Regulation (EU) No 1169/2011 provides, in its Article 7, that "food information shall not be misleading, particularly: (b) by attributing to the food effects or properties which it does not possess […] "and in its Article 7(4), that the above requirements apply to the advertising and/or the presentation of food.
Regulation (EC) No 1924/2006 on nutrition and health claims made on foods lays down the legal framework for using health claims, namely, claims which state, suggest or imply that a relationship exists between a food category food, or one of its constituents and health in the EU market.
According to that Regulation, health claims are allowed for use in the EU market only if they are authorised by the Commission, following a scientific assessment of the European Food Safety Authority (EFSA).
Health claims may be used in the labelling, presentation or advertising of foods placed on the EU market only if they comply with Regulation (EC) No 1924/2006. In particular, in its Article 3(a), the Regulation provides that the use of claims shall not be false, ambiguous or misleading.
The EU Register of nutrition and health claims lists all authorised and non-authorised health claims and it is publically available.
Regulation (EC) No 178/2002 provides a basis to ensure that the internal market in food products functions effectively and to prevent fraudulent or any other practices that may mislead the consumer. In accordance with Article 17 of this Regulation food business operators at all stages of production, processing and distribution within the businesses under their control must ensure that foods satisfy the requirements of food law, which are relevant to their activities and must verify that such requirements are met.
Regulation EC No 2017/625 on Official Controls (article 138) specifies that "measures where the non-compliance is established by the competent authority shall order the cessation for an appropriate period of time of all or part of the activities of the concerned operator and, where relevant, of the Internet sites it operates or employs".
The Administrative Assistance and Cooperation System (AAC system) is included in Title IV of Regulation (EU) 2017/625. This Regulation lays down operational rules and a standard format for the exchange of information on instances of cross-border non-compliance within the IMSOC in accordance with the power conferred on the Commission by Regulation (EU) 2017/625.
To ensure swift and appropriate coordination between different competent authorities through correct procedures (RASFF or AAC), the Commission Implementing Regulation (EU) 2019/1715 include rules for the clear distinction between non-compliances generating risks and other non-compliances.
Regarding online intermediaries such as online marketplaces, the legal framework is set by the E-Commerce Directive, which harmonises the liability exemption for the third-party content they host.
The Directive requires them to act expeditiously once they acquire actual knowledge of illegal activities or information. The Directive also prohibits imposing general obligations to monitor the information they store, or to actively seek facts and circumstances indicating illegal activity. The Commission issued a Recommendation with guidance on the measures online platforms should take to mitigate risks of being abused by their users to carry out illegal activities.
Finally, the Unfair Commercial Practices Directive aiming at protecting consumer economic interests from unfair business-to-consumer commercial practices could also be referred to by some competent authorities.
