About the Animal Health Law
The European Parliament and the Council adopted the Regulation (EU) 2016/429 on transmissible animal diseases ("Animal Health Law") in March 2016. It has been applicable since 21 April 2021.
Overall, the single, comprehensive new animal health law supports the EU livestock sector in its quest towards competitiveness and safe and smooth EU market of animals and of their products, leading to growth and jobs in this important sector:
- The huge number of legal acts are streamlined into a single law
- Simpler and clearer rules enable authorities and those having to follow the rules to focus on key priorities: preventing and eradicating disease
- Responsibilities are clarified for farmers, vets and others dealing with animals
- The rules allow greater use of new technologies for animal health activities - surveillance of pathogens, electronic identification and registration of animals
- Better early detection & control of animal diseases, including emerging diseases linked to climate change, will help to reduce the occurrence and effects of animal epidemics
- It offers more flexibility to adjust rules to local circumstances, and to emerging issues such as climate and social change
- It sets out a better legal basis for monitoring animal pathogens resistant to antimicrobial agents, supplementing existing rules and Regulations on veterinary medicines and on medicated feed
The animal health law was part of a package of measures proposed by the Commission in May 2013 to strengthen the enforcement of health and safety standards for the whole agri-food chain.
As such, it is closely linked to Regulation (EU) 2017/625 ("Official Controls Regulation"). The animal health law is also a key output of the Animal Health Strategy 2007-2013, "Prevention is better than cure".
Several delegated and implementing actshave been adopted by the Commission to make the new rules applicable. In addition to those delegated and implementing acts under the animal health law, under the official controls regulation Delegated Regulation (EU) 2022/671 and Implementing Regulation (EU) 2022/160 were also published to cover some specificities of official controls on the animal health area.
The Commission has duly consulted experts, Member States and other interested parties, EU stakeholders (e.g. in the Animal Health Advisory Committee) during the drafting of these delegated and implementing acts, in the spirit of better regulation principles.
List of animal diseases relevant for the Union intervention and categories of animal diseases and list of species and groups of species are laid down in Regulation (EU) 2018/1629 and Regulation (EU) 2018/1882.
On the new rules, a series of 5 seminars was provided to the competent authorities of Member States and of certain third countries under the Better Training for Safer Food (BTSF) initiative between 19 January and 24 June 2021, as well as a conference on 21 April 2021, also including numerous stakeholders.
These were done as video (remote) events, 3 half-day for the seminars and 2 half-days for the conference.
All of the training materials are available here.
From September 2021 to May 2022 a new series of BTSF trainings has been delivered as video (remote) events at 7 occasions.
From June 2022 the BTSF trainings are foreseen to switch back to traditional format (classroom + practical exercises).
For further information, please contact SANTE-ANIMAL-HEALTH-LAW@ec.europa.eu.
General Q&As
Regulation (EU) 2016/429 on transmissible animal diseases ("Animal Health Law") - April 2021
Regulation (EU) 2016/429 (Animal Health Law) on transmissible animal diseases is about animal diseases that can pass on from animal to animal or to humans. It provides for principles and rules for the prevention and control of such diseases in animals kept by humans, wild animals and certain animal products.
These rules consist of requirements for disease prevention, awareness, surveillance, control and eradication; biosecurity; traceability of animals and animal products; movements within the EU and entry into the EU of animals and animal products; as well as emergency measures. These rules are aimed at diseases listed in the Regulation, as well as emerging diseases.
This Regulation does not provide rules on animal welfare, although it recognises that animal health and welfare are linked and requires that animal welfare is taken into account when considering the impacts of diseases and measures to combat diseases. Other important areas such as EU veterinary expenditure, authorisation and use of veterinary medicines or medicated feed, veterinary education, official controls are dealt with in other pieces of legislation.
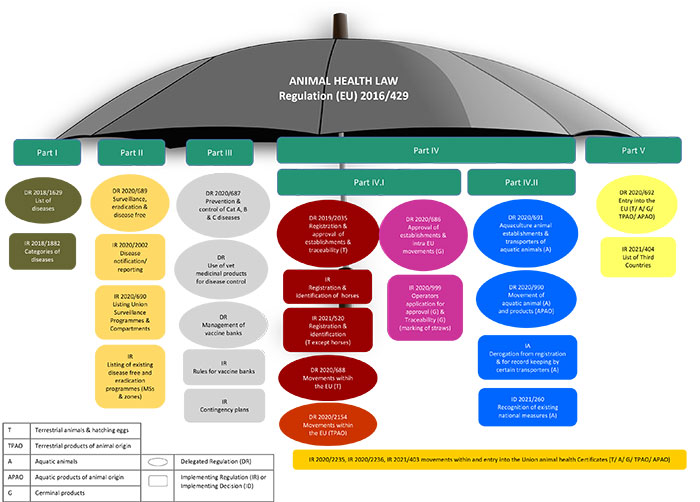
The Regulation is also complemented by a number of Commission delegated and implementing acts, covering various areas and containing further details as can be seen in the diagram below.
There are around 12 million livestock holdings in the EU. In 2019 there were around 77 million bovine, 143 million porcine animals, 74 million sheep and goats in the EU27. In 2019 just over half of the EU-27’s meat production was from pigs (22.8 million tonnes), while poultry meat production reached 13.3 million tonnes, a new high. There are about 1.6 billion heads of poultry.
Pet animals represent the second largest category of animals. There are around 120 million dogs and cats, and approximately 35 million pet birds. This law is also relevant for EU aquaculture which in 2018 production amounted to 1,32 million tonnes, with a total value of EUR 4,80 billion.
The impacts of animal disease outbreaks vary widely. These impacts can include negative effects for the health of animals and humans, the costs to farmers and related industries, the costs of dealing with disease and of business disruption, also public sector costs of disease eradication and monitoring, and changes in consumption patterns. Often, disease outbreaks also have significant impacts on international trade of animals and animal products. Finally, animal diseases may also affect wild animals, and may have detrimental effects on their populations and a negative environmental impact.
Some examples of past animal health crises to illustrate the potential scale of the impacts of animal disease outbreaks. Some of these are estimations and should be treated with caution:
- FMD (Food and Mouth Disease): The total annual impact of FMD due to direct losses and vaccination was estimated to range between US$6.5 billion and US$21 billion with an average value of US$ 11 billion globally, the majority of FMD impact occurring in China, India and Africa.
- Avian influenza in the Netherlands (2003): 30 million birds and direct economic costs of more than €150 million. A veterinarian died due to this disease. More recently, avian influenza affected 22 EU Member States in 2016-17 and 2020-21 and led to the culling of over 9 million and 12,5 million poultry, respectively, from infected establishments.
- Due to African swine fever over 700 000 pigs were culled in the EU since 2014.
Most of these rules have already existed, in one way or another, in legislation in force before the application of the Animal Health Law. Some have been around for decades, as they were essential to fight certain animal diseases and to ensure a safe and smooth internal market for live animals and their products. Where possible, those rules have been adapted, aligned and made more coherent or less burdensome.
In the event of animal diseases, veterinary services and livestock keepers would largely have to follow similar rules to current rules, for example in cases of outbreaks of foot-and-mouth disease and highly pathogenic avian influenza.
Overall, the novelty is that all the relevant elements are presented in a coherent EU harmonised legal framework, also embracing the One Health approach .

- Clear criteria are laid down to list animal diseases of concern for Union regulatory measures, as well as to list animal species subject to those regulatory measures. A new list of diseases relevant for the EU intervention has been established based on those criteria.
- Basic responsibilities of animal keepers and veterinarians are laid down in respect of the health of their animals, biosecurity measures, early detection and prevention of animal diseases, their surveillance and for animal health visits.
- Operators are to inform the competent authority about their establishments keeping animals or collecting, producing, processing and storing germinal products for registration (unless those are exempted or already approved by the competent authority). In order that the competent authorities have an up-to-date overview of the establishments, their activities, their health status and the risk they may represent, operators are to keep a number of relevant records.
- In terms of notification of animal diseases, operators must notify the competent authority where there are any reasons to suspect the presence in animals listed epidemic diseases. Operators must notify also suspicion or detection of other listed diseases or abnormal mortalities and other signs of serious disease or significant decreased production rates with an undetermined cause.
- The Animal Health Law also introduces a new system for Union notification and reporting of animal diseases, so–called Animal Disease Information System (ADIS).
- Other new elements are related to extending the legal possibility for measures for the prevention and control of emerging and listed diseases and health measures in wild animals.
- As a part of the overall on-farm surveillance system another new element are Animal Health Visits of the veterinarians on farms. The Regulation provides such visits as a complementary measure to other systems of surveillance and control, including to the measures by competent authorities. Animal health visits will be put in place by operators. These visits can also provide operators with relevant advice on biosecurity and other matters related to animal health.
While the majority of the new rules remained the same or similar to existing ones, newly harmonised requirements are now laid down for several more animal species or groups of terrestrial animals and germinal products to address certain risks (e.g. captive birds, carnivors). On the other hand obligation to certify certain movements were abolished to reduce administrative obligations (e.g. certain germinal products, bumble bees from environmentally isolated production establishments). The same is true for the movements of aquatic and most importantly aquaculture animals.
Past experience with the spread of highly contagious animal diseases have shown that assembly operations represent a major risk for spreading animal diseases. Therefore, strict animal health requirements remain for such risky operations.
As regards entry into the Union, the overall system remains largely as it is. A key element in this area is that the animal health requirements for the entry into the Union of animals, germinal products and products of animal origin are to be as stringent as the animal health requirements applicable to movements within the Union or they are to offer equivalent guarantees.
Despite the conservative approach, the new rules include remarkable changes on the requirements for the entry into the Union of certain specific commodities such as casings and composite products. At the same time, as consequence of the new listing and categorisation of diseases, certain requirements for the entry into the Union of animals and products have been updated and aligned with the requirements applicable within the Union.
Export from the Union is in animal heath legislation, similarly to what already exists in the General Food Law, regulated for the first time at the Union level under the Animal Health Law. In this regard, the new rules are basic, mainly intended to provide for the responsibility for Member States to ensure that commodities exported from the Union are safe and do not pose an animal health risk for the place of destination.
Yes, this Regulation introduces a special chapter on non-commercial movements of pet animals. These new rules take over the rules from the existing Regulation (EU) No 576/2013 on the non-commercial movements of pet animals. However, there is a transitional period until 21 April 2026, during which Regulation (EU) No 576/2013 will continue to apply. As from that date on, rules of a special chapter on non-commercial movements of pet animals of the Animal Health Law will apply.
The Regulation applies from 21 April 2021. Most rules that applied before its entry into application, are repealed from that date and no longer apply. There are, however, several exceptions. For example, for non-commercial movements of pet animals, the Regulation has a longer, 10-year transitional period. Those rules apply until 2026.
As regards certificates for movements between Member States and for entry into the Union of animals, certificates, which applied before the entry into application of the new rules, can be used for a few more months after 21 April 2021.
Certificates, which applied before the entry into application of the new rules, for the movement between Member States of animals and animal products will start to be used on 17 October 2021.
Certificates, which applied before the entry into application of the new rules, for the entry into the Union of animals can also be accepted until 20 October 2021, provided, that they were signed before 21 August 2021.
Information material
The European Commission services have prepared a set of information material to support the implementation of the AHL and to help to raise awareness on different parts of the new EU animal health rules.
This information material consists of a poster, a leaflet, a short video as well as nine thematic factsheets. They are available in all EU official languages.
The poster, the leaflet and the video provide general information on the new animal health rules.
The factsheets provide more focussed information on certain animals, products or activities and are targeting specific groups.
Evaluation of the Animal Health Law
According to Article 282, the Commission must evaluate Regulation (EU) 2016/429 together with the delegated acts and submit the evaluation results in a report to the European Parliament and the Council by 22 April 2026.
This comprehensive evaluation aims to assess the effectiveness, efficiency and proportionate, coherency and EU added value of the AHL (the basic Regulation) and the delegated acts adopted on its basis. It is an essential process to assess that the legal framework delivers the necessity tools for the competent authorities, operators, and other stakeholders to effectively prevent and control animal diseases within the EU single market.
The evaluation will also determine whether the framework is easy to understand and apply building confidence in animals and their products in the single market. Potential for simplification and proportionality of benefits and costs will be looked at with particular attention.
More information could be found on the "have your say"-page.
In March 2024, the Commission launched a Call for Evidence, receiving feedback from 942stakeholders across the EU, which forms the basis for ongoing discussions on how to improve the Animal Health Law and is summarised in the Factual summary of the call for evidence of the evaluation of the Animal Health Law. More information on the recent evaluation process, consultation can be found on the "have your say"-page.
The summary report of this consultation, which highlights key stakeholder feedback was discussed by the Animal Health Advisory Committee during its meeting on 5 September 2024. The minutes of this meeting, including the key points and outcomes from the meeting can be found Animal Health Advisory Committee page.
To support the evaluation process, an Evaluation Study was launched in May 2026. The study is coordinated by a consortium led by Ecorys and involves a targeted consultation with key stakeholders, including:
- National competent authorities
- Members of the Animal Health Advisory Committee
This consultation will use mix of surveys, focus groups, case studies and interviews to gather comprehensive input from stakeholders in the EU and non-EU countries. For further details on the study and consultation activities, please contact the evaluation team directly at evaluationAHL@ecorys.com.
